GROUP MEMBERS:
Saxon Aden
Akram Khan
Purpose and Criteria for success:
1) Construct a laser from a blu-ray DVD player optic drive by removing the blue diode and creating a working blue laser.2) Using the blue diode laser, produce enough energy to burn a match and pop a balloon.
3) Be able to measure the heat produced by the blue diode laser and compare with other laser diodes.
Materials:
•Blu-ray
DVD diode (ebay.com)
•Soldering
Iron
•Solder
•Wire
strippers
•Wire
cutter
•4.3
Ohm resistor
•Diode
housing with lens
•1
watt blue laser diode driver (400-405nm)
•Knife
•Small
flathead screw driver
•Battery
- DC Power Supply (Thank
You Professor)
•Alligator
Clips (Thank You Professor)
•Green
Diode (amazon.com)
•Red
Diode (amazon.com)
•Thermal
Couple (Thank You Professor)
•Temperature
Meter (Thank You Professor)
Procedure:
 |
| Optic slide of the DVD blu-ray player diode. |
 |
| Pry the diode with heat sink out. Caution: Has super glue, it will pop out. The blue diode will be marked with blue mark, other diode is not marked and represents the red/infrared diode (not needed). |
 |
| Wires that are connecting to diode pins need to be cut |
 |
| Diode with heat sink |
 |
| Cut heat sink away from diode. Caution: do not cut diode. |
 |
| Heat sink and diode removed from eachother |
 |
| Blu-ray Diode |
 |
| Remove the wires from the pins that need to be de-soldered |
 |
| Diode needs to be pressed into a housing. |
 |
| Diode with housing, (unclamped) |
 |
| Outcome of the pressing of the diode inside the housing components |
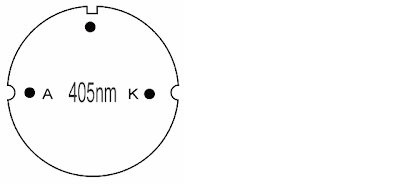 |
| Note markings on the diode. Left semicircle marks represents the anode while the right semicircle markings represents the kathode. |
 |
| Soldering 4.3 Ohm resistor to the anode of the diode. |
 |
| Resistor to the anode, and wire to the kathode ensuring they are the same length. |
 |
| driver component of the laser. purpose is to limit power |
 |
| Solder driver onto housing component. Following the picture; anode connects with southern connector of driver, kathode connects to northern connector of the driver. |
 |
| Solder wire to reverse side of driver which will become the positive connector. Negative goes to spring part of the driver. |
Measuring temperature of the heat produced by the lasers.
 |
| Apparatus of the thermal couple portion of the experiment |
 |
| Red diode |
 |
| Green diode |
 |
| blue diode |
DATA AND ANALYSIS:
Conclusion
Green
Laser had limiting power output, resulting in high error and skewed data.•Theoretically, the green diode laser should output power proportional to the amount of temperature reading.
•Much of the power put into the green laser was lost through heat and gave a small and unimpressive temperature reading.
•The blu ray DVD diode was susscesfully
used to create a working laser. Not only was the laser working, there was
enough power that was put into the laser that produced enough heat to be able
to burn some materials such as a match.
•The heat that was produced from the laser
was also calculated to show how much heat the laser was producing and how the
rate of temperature increased as time increased with a constant level of power.
•The laser was also compared to store-bought lasers which helped compare the power level that it was being given to
the lasers. Based on the data and information gathered, much of the power that
was being put into the laser was being also lost as heat, especially for the
green laser since there was a limiting factor preventing the laser from
producing any heat. There was uncertainty given for that and based on the uncertainty it can be verified that the more power that was given to a laser, the higher the temperature would increase as time progressed.






































